Benzodiazepines (BZDs) are among the most commonly prescribed
drugs in the U.S. Data for 1996-2013 show a 67% increase in adults filling BZD
prescriptions. During the same period, the total quantity of BZDs filled
increased 3.3-fold and overdose mortality involving BZDs increased 5.3-fold. An
estimated 75% of deaths involve the use of opioids in addition to BZDs, likely
due to worsening of opioid-induced respiratory depression by BZDs. Compounding
these concerns are the use of non-Food and Drug Administration (FDA)-approved
BZDs.
BZDs in the U.S. fall into essentially two groups: FDA-approved and
non-FDA-approved compounds (Figure 1). Non-FDA-approved BZDs can be delineated
into two further groups: BZDs approved in other countries and designer BZDs
(DBZDs) (often called synthetic, novel, or novel psychoactive substances).

The latter can be further divided into BZDs prescribed
outside the U.S. and true designer BZDs (DBZDs) not approved for medical use
anywhere in the world. DBZDs are often structurally very similar to approved
BZDs (exceptions are flutazolam and ketazolam). A few examples follow.
Cloniprazepam is metabolized into clonazepam by the removal of the
cyclopropylmethyl group (circled in orange). Clonazolam can be considered a
hybrid of clonazepam (functional groups circled in blue) and alprazolam
(triazolo group circled in purple). Similarly, flubromazolam is a triazolo
analog of flubromazepam (difference circled in red). Flubromazepam only differs
from phenazepam by the substitution of the chlorine group by fluorine (circled
in green). Adapted from Marin et al.
Several BZDs, such as etizolam and
phenazepam, are approved in a handful of countries but are often misused in the
U.S. and other countries. Etizolam was first introduced in Japan and is also
approved in India and Italy. Phenazepam was developed in the Soviet Union and is
still approved for use in Russia and other former Soviet states. DBZDs have
often been described in the scientific or patent literature as compounds that
were explored by pharmaceutical companies but never developed into drugs. They
are not approved for medical use anywhere in the world.
Chemically,
non-FDA-approved BZDs maintain much of the primary structure of FDA-approved
BZDs. However, they are modified from this primary structure by the addition
and/or deletion of certain functional groups. Some DBZDs are hybrid molecules of
different FDA-approved BZDs. They may also be either potent metabolites of
FDA-approved BZDs or compounds metabolized into FDA-approved BZDs. For example,
the DBZD clonazolam may be considered a hybrid of alprazolam (technically a
triazolo BZD) and clonazepam, both of which are commonly prescribed BZDs. A
newer abused DBZD, flualprazolam, is based on alprazolam with an additional
fluorine group. These additions and substitutions at specific locations have
generally predictable effects on BZD activity. (Figure 2,
online)
TRENDS AND PUBLIC HEALTH CONCERNS
Today, many
non-FDA-approved BZDs are sold via illicit marketplaces such as the darknet
(also called dark web or deep web), a part of the internet that is not indexed
and can only be accessed using special browsers. They’re also sold on other
obscure websites that label these compounds as research chemicals or legal
alternatives. This is concerning as data are limited on the clinical effects and
pharmacologic characteristics of DBZDs.
One recent study retrospectively
evaluated multiple case reports and found that the clinical effects of DBZDs and
etizolam were generally consistent with sedative-hypnotic toxidrome, as would be
expected for BZD derivatives. Severe effects, however, were uncommon . Thus,
clinically suspecting non-FDA-approved BZD use may prove itself difficult
because there seems to be no distinct difference from the sedative-hypnotic
toxidrome seen in FDA-approved BZDs. Further, many of the cases in which
non-FDA-approved BZDs have been detected were in conjunction with other classes
of commonly abused drugs. It is important to note, however, that single agent
exposures of non-FDA-approved BZDs are on the rise.
Illicit markets often
sell non-FDA-approved BZDs under the guise of FDA-approved BZD names. For
example, flubromazolam and etizolam have been detected in fake Xanax tablets
instead of alprazolam. As the pharmacological effects of these compounds are
largely unknown, this trend poses dangers not only for recreational users who
believe they are purchasing a familiar BZD, but also potentially for unwary
patients.
The latter group may not be at risk as an unintended side effect of
a potential restriction on BZDs, which in general have the risk for physical
dependence and addiction. A clamp down on BZD prescribing could lead to patients
seeking these medications through illicit channels. This concept is not new and
parallels the ongoing U.S. opioid crisis.
CURRENT MONITORING METHODS
AND THEIR LIMITATIONS
Most data on reported exposures and trends of
non-FDA-approved BZD use in the U.S. are reported retrospectively by large
institutions such as the National Poison Data System and the Drug Enforcement
Administration. Internationally, early warning systems exist through the United
Nations Office on Drugs and Crime and the European Monitoring Centre for Drugs
and Drug Addiction.
The U.S. does not have a regularly used, centralized
reporting system, and real-time monitoring is often lacking. However, even
efforts that capture information more prospectively, such as the STRIDA project
in Sweden, still underestimate the total number of DBZD-related intoxications
(5). Thus, usage and outbreaks are often underreported and not well defined
until communities begin to experience their effects. Notably, once a DBZD has
been regulated as a controlled substance, often one or more new (non-regulated)
DBZDs make their way into a community(Figure 3). This continues to be a legal
game of whack-a-mole in which regulatory and enforcement agencies, monitoring
institutions, clinical laboratories, and healthcare providers are always playing
catch-up with drug outbreaks.
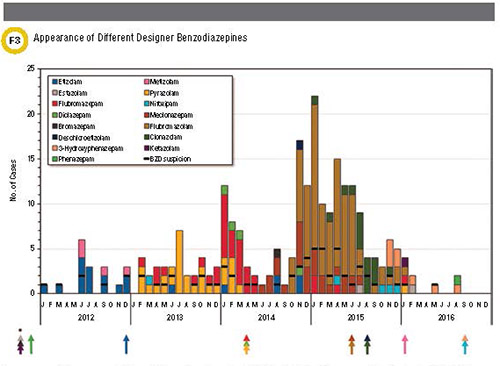
Appearance and disappearance of different designer
benzodiazepines (DBZDs) and etizolam/phenazepam in Sweden during 2012-2016
analyzed as part of the STRIDA project. Disappearance of a DBZD often coincides
with the time of controlled substance classification (represented by the
arrows). As indicated by the asterisk, some compounds were classified before
2012. Reproduced from B?ckberg et al.with permission from Taylor &
Francis.
SUGGESTIONS TO IMPROVE MONITORING
In order to
improve monitoring of non-FDA-approved BZDs, a few concepts are essential.
First, monitoring should occur in real time through a centralized reporting
system. Centralized, free-of-charge testing for providers who suspect designer
drug use is likely a valuable adjunct. Such testing was successfully implemented
in the STRIDA project.
In addition, this information should be continuously
updated. Making a real-time database useful requires a standardized way of
continually disseminating the information, reaching not only toxicological and
laboratory experts but also interested health providers. A local, real-time
heatmap of identified DBZDs and designer drugs in general would provide
invaluable data to providers and laboratorians.
Finally, monitoring should be
proactive. That is, can we predict which novel DBZDs will appear next within a
community or location? For example, could we take selected data from patents,
scientific literature on BZDs that were never developed into drugs, current data
on approved and non-approved BZDs, timelines of newly identified DBZDs in
communities, and integrate this information to make predictions, potentially
through the use of artificial intelligence?
For example, a current trend is
to produce triazolo analogs of FDA-approved BZDs or DZBDs: clonazolam is the
triazolo analog of the FDA-approved clonazepam and flubromazolam is the triazolo
analog of flubromazepam, a DBZD (Figure 1). Another trend is to produce various
modifications of etizolam.
Monitoring could also take the shape of gathering
data from social media where DBZDs are discussed, such as Reddit and Bluelight,
as well as tracking items for sale on obscure internet sites and the dark
web.
In aggregate, standards of potential new DBZDs could be produced
proactively, not unlike the Psychoactive Surveillance Consortium and Analysis
Network project for surveillance of synthetic cannabinoids. These compounds
could then be added to confirmation assays ideally before, or soon after, the
start of an outbreak. A comprehensive, proactive approach would enhance
preparedness and detection both on a clinical and laboratory
level.
CHALLENGES IN IDENTIFICATION AND
INTERPRETATION
The good news is that immunoassays for BZDs generally
have good cross-reactivity for non-FDA-approved BZDs. The exceptions are DBZDs
with atypical structures such as flutazolam and ketazolam (Figure 1). However,
as these cross-reactivity studies generally test parent compounds, it is
possible that primary drug metabolites might not cross-react using
antibody-based assays. This problem has been suspected in the case of
flubromazepam.
However, when a preliminary positive sample that contains a
non-FDA-approved BZD is analyzed for confirmation by mass spectrometry (MS), it
will likely be negative, as many targeted BZD confirmation assays do not test
for these compounds. This may lead to the incorrect interpretation of a false
positive immunoassay-based BZD result. A study in Sweden found that 40% of
presumably false-positive BZD results actually contained a non-approved
BZD.
Due to the rapidly changing landscape of DBZDs, clinical and forensic
toxicology laboratories face an almost impossible task to keep their targeted
methods up to date. Clinical laboratories have the option of using untargeted
data acquisition by high-resolution MS, for example using a
quadrupole-time-of-flight (QTOF) instrument. This approach offers fast method
development, and novel DBZDs can be preliminarily identified based on the highly
accurate exact mass and isotope pattern and later confirmed with a reference
standard. Additionally, a laboratory could analyze data retrospectively for
compounds that were not in the library during the time of analysis. However,
high-resolution instruments remain costly.
Another challenge for analytical
identification—especially in urine—is the limited knowledge of DBZD metabolites.
In vitro human liver microsome-based and single-subject self-administration
studies have shed some light on which metabolites are likely targets for
detection. Targeting the parent compound may be sufficient to detect some DBZDs.
For example, pyrazolam is mainly excreted as unchanged parent drug. However, for
other DBZDs, only metabolites may be detectable in urine. For example, < 0.5%
of etizolam is excreted as unchanged parent drug. Moreover, such studies lag
behind the first reported use of the novel DBZD.
Lastly, as new DBZDs appear
quickly, there is a considerable lag time in the availability of reference
standards. As mentioned above, predicting novel DBZDs and proactive synthesis of
standards would help circumvent several of the aforementioned limitations.
In
addition to incorrectly interpreting positive immunoassay results with negative
confirmations, clinical laboratorians must navigate other pitfalls in
interpreting MS results. For example the metabolite of the DBZD cloniprazepam is
the FDA-approved drug clonazepam (Figure 1); thus, one could falsely interpret
the use of cloniprazepam as clonazepam use. Similarly, the metabolites of
diclazepam are the pharmaceutical drugs delorazepam, lormetazepam, and lorazepam
(although only lorazepam is FDA-approved).